What happens when you get a group of testate amoebae together, after they’ve been filmed in a Smith professor’s lab, and “interview” the microbial actors? It turns out that they’re prepared to reveal quite a bit about themselves. What follows is Insight’s look at the ultimate microbiology research subject.
/ Published June 20, 2014
In biological nomenclature, most any living thing you’ll observe with your naked eye in your lifetime is a eukaryote: an organism whose cell(s) have a nucleus that houses its genome (from the Greek eu- for well, karuon for kernel). And yet most of eukaryotic life on earth can’t be seen by humans without the aid of a microscope: of 75 major evolutionary lineages (or clades) of eukaryotes, only three—animals, plants, and fungi—are not exclusively microbial. What’s more, most of those that are microbial haven’t been studied.
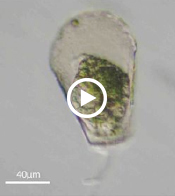
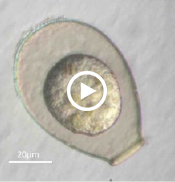
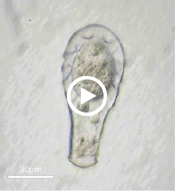
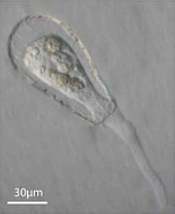
Enter Laura Katz, the Elsie Damon Simonds Professor of Biological Sciences at Smith, and the research team at Katz’s Smith lab (see sidebar), who work to fill in the blanks of evolutionary history and theory by studying the biodiversity of eukaryotic microbes. Katz’s team currently focuses on microbes drawn from regional aquatic environments, including coastal ciliates (microbes with fringe-like external organelles called cilia) and freshwater testate amoebae (irregularly shaped microbes with shells, or tests), documenting patterns in community population and genetic inheritance, as well as refining species classification based on DNA analysis. In doing so, they hope to assess the biodiversity and phylogeography of these species.

Laura Katz
“We take photographs of these individual cells, and [then] we isolate their genome,” says Katz, describing the balance struck between a visual record, or “voucher” of what her team observes with the much more detailed portrait offered by cell DNA. “We can’t observe their behavior over years,” since many of the organisms the Katz lab studies live only a few weeks in the lab, “but at least as a starting point we can decode their genetic information.”
One facet of the Katz lab’s visual record-making includes a series of short films chronicling particularly striking behavior in testate amoebae, such as possible sexual activity and the presence of possible parasites or symbionts within the amoeba’s shell. As a nod to the team’s ingenuity, Insight decided to put a new slant on the topic of microbial biodiversity: What happens when you get a group of testate amoeba together after they’ve been filmed and “interview” the microbial actors? What follows is our attempt at an answer.
This fictional interview is based in part on real interviews with Laura Katz, Cameah Wood ’15 and Hana Kanee ’15.
Interviewer: Hyalosphenia papilio, you and your co-stars recently appeared in a series of documentary portraits by members of the Katz lab, which one critic has called a “haunting tone poem of eukaryotic solitude.” Tell me, how would you characterize the Katz lab directorial style?
H. papilio: The Katz lab, as a whole, is definitely influenced by the cinema verité style — members of the lab embrace the sublime and facilitate us to be completely natural and improvisatory when we’re in the dish. It’s enough to make you forget that you’re in a contrived environment.
This seems to be a big moment for independent microbial cinema. What’s your take on that phenomena?
H. Papilio: Oh, this question.
Nebela tincta: I’ll take this one. I think there’s a popular conception that this sort of work is brand new. I understand where that comes from: for years, so much of this work has been so star-driven; if I see another magazine cover with E. coli on it, I swear, I’ll scream. That’s what’s been studied predominantly in the recent past because it’s easy. E. coli has a blown-out-of-proportion bad-microorganism rap in the human world, so it might be surprising for me to say this, but part of why it’s so famous in our circles is that it’s a go-along, get-along kind of microorganism; it’s relatively easy to culture and keep alive in the lab. But only for the past 50 years or so has that focus shifted predominantly onto star organisms and away from the sorts of biodiverse ensemble casts that make up any habitat, be it the human body or someplace in the natural world. It’s not a new thing or a fad that lesser-studied organisms like Hyalosphenia elegans, H. papilio and myself are now being looked at; it’s an enduring scientific concern that’s resurging in part because of the increased availability and efficiency of DNA analysis tools that tell you who we are as organisms, coupled with photographic evidence and films, of course.
Hyalosphenia elegans: It’s bittersweet, really: the films exist because we won’t for long enough.
N. tincta: Yes. Yes, that’s exactly right. We don’t stay alive long in the lab. But also, I was going to say: look at Lena Dunham’s second film Tiny Furniture. Maybe that never would have been made if she hadn’t been able to shoot it on a cheap digital camera and —
H. papilio: Is she the best example? I mean, I’m a fan, but it’s not as though she invented —
N. tincta: No, I know, you’re right. That’s a recent example, but still the increasing availability of digital cameras has enabled a lot of independent filmmaking. And although it’s an imperfect analogy, it is sort of similar to what happened with the study of microbial biodiversity, where the increased availability and efficiency of DNA sequencing tools, still coupled with microscopic cinematography, of course, has enabled scientists to study more organisms with greater accuracy. So now there’s an increased ability to look at ensemble microbial casts in a given environment, as I’ve said, rather than making study after study of lab stars.
Quadrella symmetrica: But all microbes are relatively understudied. And eukaryotic microbes present a very rich, mostly untapped resource.
N. tincta: That’s right.
Quadrella symmetrica, you’re absent from this film series, but you’re still a hot topic in the Katz lab. Why the omission?
Quadrella symmetrica: I’m not sure that’s permanent. Like the other testate amoeba studied, I may be one morphologically identifiable species (and really, with a shell like an Escher print, how could you miss me?) or I might actually be one of several subspecies. The thing about these films — with all due respect to my lab cohorts, who’ve starred in them — is that, as the researchers themselves will tell you, they’re just a record. When you get down to the genetics level — which is much easier and faster to do nowadays, compared to back when you only had microscopes — not all the genetic information from the sequenced genome matches across samples of other microorganisms in my species classification. So when I do get filmed, for example...
H. papilio: [rolls nucleus]
Q. symmetrica: ...lab members will want to take footage of each sample of my species-mates as they sequence their genomes, so that if variation is found among them, they’ll provide a visual reference point to go back to.
Let me direct this next question to the Hyalosphenia or Nebela genera: Who are these flagellates we can see inside your tests? And what are they doing there? They’ve been the unexpected scene-stealers of this series, you have to admit.
H. elegans: They’re either helpful, harmful, or neither. We can tell you that much.
H. papilio: The Katz lab is trying to figure out who they are. Of course, it’s not as though the few of us who have been discovered with flagellates in their tests think of them in terms of classification. But even if we did, we wouldn’t tell you, and we don’t want to give away any hints as to their function; beyond the lab, people can go see the films and make their own conjectures.
All right — thanks for that. Now, why do you think work like this, on organisms such as yourself, is so important? Why are you excited to be part of this work?
H. papilio: Well, there are plenty of reasons. We’re being studied by a great team. For the bog-dwellers like myself, I’m really proud of the contribution we might make down the road as the climate changes; bog climates are very delicate, moss varieties vary even among closely neighboring bogs, so giving the team a window into who among us lives there now could provide a benchmark for changing climate conditions as the population changes — that is, after I’m gone. I won’t live to see that kind of development, but I can still be proud of it.
Q. symmetrica: I think H. papilio is onto something important here, particularly with the idea of pride in contributing to insight beyond ourselves. I’m a bog-dweller also, but the team is also doing this work on coastal ciliates. In pedagogy and biological research, from what I understand, there’s a commonly held principle that whatever changes an organism undergoes during its life isn’t heritable in the next generation. So if you’re a human, say, and you lose part of a limb and then have offspring, your children won’t have the limb pattern you developed later in life; they’ll have the limb pattern similar to the one you had when you were born. Or, they’ll be born with some variation that doesn’t have to do with your lost limb. But you look at ciliates, and that goes right out the window. Professor Katz gives the example of performing microsurgery on a ciliate: say, for example, you were to cut a slit in one side so that it then has two mouth-like openings after self-healing. Then its offspring will also have two mouth-like openings, which is completely at odds with what most of biological evolutionary theory tells us.
H. elegans: Tell them about the weird chromosomes that the humans don’t know about.
Q. symmetrica: Oh, and in terms of encoding genetic information, where a human will inherit 23 chromosomes from one parent and 23 from another, and so have a total of 46, a ciliate has 200 that it passes on to its offspring but for itself it has 25 million. That’s almost nine times and 543,478 times what humans have, respectively, not to mention that the latter is about 2,717 times the former, instead of twice the former.
N. tinca: I feel like we’re getting off topic.
Q. symmetrica: No, this is precisely on topic, because the whole wider significance of this research, these films, is that the fewer biological entities you study, the more limited your conception of biological principles is going to be — and in the case of eukaryotes, like us, the overwhelming majority haven’t been studied much in terms of diversity, in terms of behavior, in terms of evolutionary lineage.
OK, we should probably start wrapping up. Anything else you want the folks reading at home to know?
H. papilio: That the small world is big. It’s bigger than you know.
H. elegans: If you look at the third film of the series, you get an especially nice view of my shell dimples.
One more thing. I hate to ask this question, but your fans will want to know: how’s your love life?
H. elegans: I know there’s already been a lot of speculation about this, what with the extended scenes between H. papilio and myself. People love speculating these days about what goes on among the Hyalosphenia and Nebala genera. And while I realize that such distinctions can be important to the public, I prefer not to comment on our rumored meiosis capacities at this time. As H. papilio suggested earlier on the topic of flagellates, I feel that this kills the imaginative possibilities left to the audience. I can say that we’re very good friends.
H. papilio: No comment.
FINIS